 |
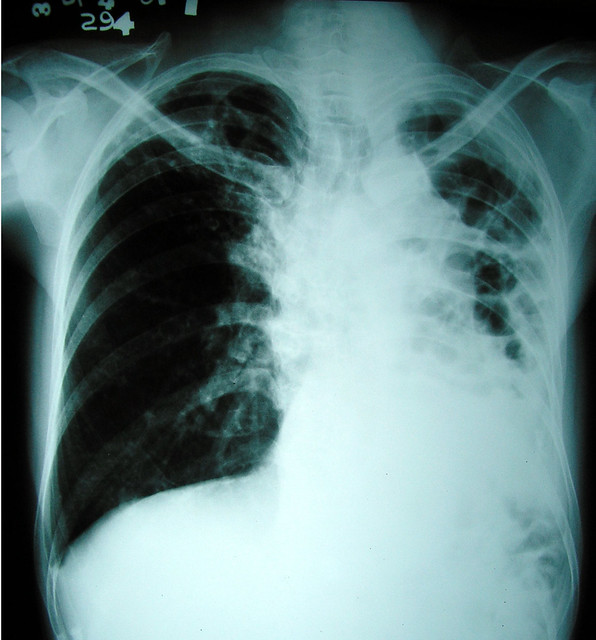
| This is an X-ray of a lung with advanced fibrosis. Courtesy of mjagbayani. |
According to court documents, Genentech developed Actimmune which the Food and Drug Administration later approved to prevent infection in patients suffering from chronic granulomatous disorder (CGD). InterMune then purchased the rights to develop and market Actimmune and conducted studies to determine whether the drug could treat patients patients suffering from idiopathic pulmonary fibrosis (IPF), a painful disease of the lungs without a known cure. The Plaintiffs alleged that the defendants knew that Actimmune was not effective to treat IPF, but nonetheless published articles that it was effective and made representations to doctors, investors and the public about Actimmune’s efficacy in treating IPF. Eventually, the FDA’s ultimately refused to allow InterMune to continue to test Actimmune as a treatment for IPF and the patients felt duped.
As related to the current case, the Plaintiffs argued that the Defendants violated the California Unfair Competition Law (UCL) by marketing the product off of its label in a way that violated either the Food, Drug and Cosmetics Act or California's Sherman Food, Drug and Cosmetics Laws and that this violation caused the consumer's harm. As the court explained:
The shortcoming in the consumer plaintiffs’ pleadings is simple: all of the consumer plaintiffs fail to allege that their doctors believed that Actimmune was an effective treatment for IPF “as a result of” defendants’ off-label promotion of Actimmune. [Notably,] none of the plaintiffs allege that their doctors believed that Actimmune was an effective treatment for IPF “as a result of” the marketing information conveyed by defendants.Judge Patel dismissed the case without leave to amend ending the lawsuit. The case is In re Actimmune Marketing Litigation, No. C 08-2376 and the opinion is below the jump.
Here is the opinion:
In Re Actimmune Marketing Litigation
C1D8E931C4
ReplyDeletekiralık hacker
hacker arıyorum
belek
kadriye
serik
54857AAF0F
ReplyDeleteTakipçi Satın Al
En Güvenilir VPN
En İyi Animasyon Filmleri
TT İzlenme Hilesi
Yabancı Film İzleme Siteleri
TT Beğeni Hilesi
UC Satın Al
Google Yorum Satın Al
Oyun Önerileri